GE醫療HyClone細胞培養產品標簽變更通知
尊敬的客戶:
感謝您一直以來對HyClone細胞培養產品的信賴與支持。隨著HyClone細胞培養產品成為 GE醫療生命科學部的一部分,為了給您提供更加準確的產品信息和更加優質的服務,我們計劃對HyClone細胞培養產品的標簽進行變更,具體變更內容如下:
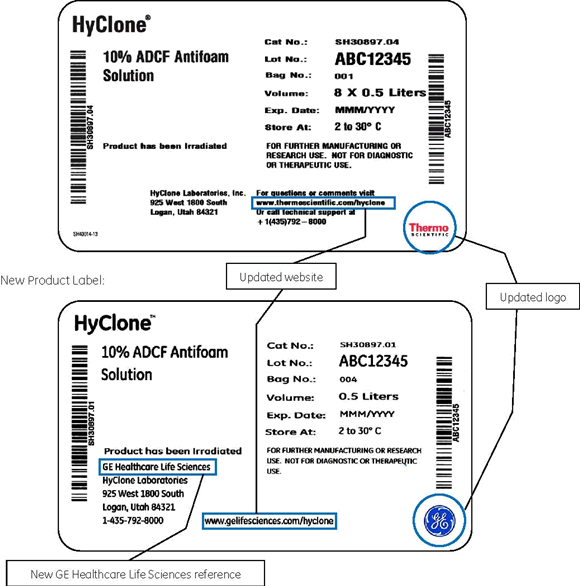
2014年 7月 15日前生產的產品依然使用原有 Thermo Scientific logo;對于 2014年 7月 15日起生產的產品,包括血清、液體和干粉培養基和工藝液體等,將會使用新的 GE醫療公司 logo,涉及變更的項目包括:
● 產品標簽
● 產品包裝
● 報價單、發貨單、訂單確認的抬頭
● 產品分析報告(COA)、產品適用性認證(COS)及其它類似項目
此次變更僅涉及HyClone細胞培養產品的標簽,對于生產加工工廠、生產工藝和流程、產品質量控制等沒有任何改變,HyClone細胞培養產品的質量和服務不受任何影響。同時,確保此次業務整合平穩順利地進行,并一如既往地為客戶提供高品質的細胞培養產品和服務也是我們的首要工作;也希望您繼續地支持HyClone細胞培養產品。
此次標簽的變更暫不涉及中國工廠生產的培養基產品,對于這部分產品,后續如有任何變更信息,我們會提前進行通知。
為了讓您對此次標簽的變能有更直觀的認識,我們在下面附上標簽更新后的產品標簽、更新前后產品分析報告(COA)的變化,供您參考。
了解更多有關HyClone的產品信息,請訪問:https://www.gelifesciences.com/HyClone
LABEL CHANGES
Current Product Label:

SAMPLE- CURRENT CERTIFICATE OF ANALYSIS
|
Product:CDM4CHO™
− L-Glutamine |
Lot#: XXX123456Catalog #: SH30558Expiration Date: MMM/YYYY
======================================================
TestSpecification Units Results
|
Appearance |
Clear solution |
|
Clear solution |
|
pH@ 20-25°C |
7.0 -7.4 |
|
7.1 |
|
Osmolality |
280 - 320 |
mOsm/kg |
287 |
|
Endotoxin |
≤10£ |
EU/mL |
< 0.1 |
|
Sterility Testing |
|
|
|
|
Bacteria and Fungi |
No Growth |
|
No Growth |
|
Growth Promotion |
|
|
Satisfactory |
=========================================================
Cell growth was assessed over a minimum of three subculture generations. Cell cultures are observed for evidence of nutritional deficiency, cytotoxicity, or morphological aberrations.
Cell Lines used: Suspension CHO-K1 Cells.
Quality Control Department Date.


SAMPLE- NEW
CERTIFICATE OF ANALYSIS
Product: CDM4CHO™−L-Glutamine
Lot #: XXX123456Catalog #: SH30558Expiration Date: MMM/YYYY
=========================================================
Test Specification Units Results
=========================================================
|
Appearance |
Clear solution |
|
Clear solution |
|
pH@ 20-25°C |
7.0 -7.4 |
|
7.1 |
|
Osmolality |
280 - 320 |
mOsm/kg |
287 |
|
Endotoxin |
≤10 |
EU/mL |
< 0.1 |
|
Sterility Testing |
|
|
|
|
Bacteria and Fungi |
No Growth |
|
No Growth |
|
Growth Promotion |
|
|
Satisfactory |
Cell growth was assessed over a minimum of three subculture generations. Cell cultures are observed for evidence of nutritional deficiency, cytotoxicity, or morphological aberrations.
Cell Lines used: Suspension CHO-K1 Cells.
Quality Control Department Date.
- 百蓁生物邀您共赴CBI 2025生物醫藥創新博覽會
- 明美光電首屆蘇州經銷伙伴大會圓滿舉辦
- 中國醫科大學功能超聲fUS成像儀ICONEUS ONE成功裝機
- 上海優耳儀器科技有限公司成立20周年答謝函!
- 覃思科技成為丹麥DeltaPix顯微成像產品中國區代理商
- 易科泰創新實踐國家發改委“百城千企”課題
- 盧湘儀離心機亮相央視,國產科研利器賦能經濟發展
- 贊德公司關于美國Med associaties中國獨家代理聲明
- MCE推出AI藥物篩選平臺,實現數千萬的分子快速篩選
- 迪必爾入選2025關鍵技術研發計劃"合成生物學"項目
- 美國國立衛生研究院宣布停止對僅動物研究的資助
- 智聽自然,聲動未來:沃德精準亮相"聲景中國"研討會
- 赫西智能高速冷凍離心機亮相央視"革新人工催產技術"
- 易科泰榮膺“SFAA 植物工廠應用十大優秀企業”稱號
- 百蓁生物推出一站式高精度HDX-MS分析服務
- 6月26日潔特生物直播預告:國產化替代解決方案
- 杭州欣友生物適合邊緣孔拍照的96孔玻底板新品上市
- Labubu陪你'擴增'快樂!PCR 8聯管促銷熱力循環中
- 杭州欣友生物激光共聚焦專用cellvis 32mm玻底皿上市
- 耐思SCI引用量突破3800篇,總影響因子2.27w+
- 潔特生物推出多種規格細胞刮刀,助力保護細胞活性
- 康寧公布2025年第一季度財務業績,強勁表現超出預期
- 關稅波動情況下科茲莫關于穩定供應及品質承諾的聲明
- BIOLOG 推出新品益生元板和厭氧菌培養基優化板
- ibidi micro-Insert 3D微孔插件及培養皿新品來襲
- MD官方商城上線52款試劑耗材新品,限時好禮相送
- 賽默飛開工返校季促銷:高品質細胞培養耗材尊享價
- 耐思一次性筆式注射器獲得加拿大醫療器械許可證
- 康寧公布2024年第四季度及全年強勁財務業績
- ibidi新品:應用于高分辨率顯微鏡成像的6孔培養板
Copyright(C) 1998-2025 生物器材網 電話:021-64166852;13621656896 E-mail:info@bio-equip.com






